I believe that autism has been ‘discovered’ in the wrong order. Autistic thought, loosely labeled as the broader autism phenotype (BAP), is a perfectly normal and reasonable example of neurodiversity, within the normal range of human thought. I see autistic disabilities as being driven by sensory integration issues to which those who fall within the BAP are more vulnerable. In this paradigm it is reasonable to expect the medical and psychiatric professions to ‘discover’ ASD in reverse order of severity, i.e. defining first the more severe disabilities, then lesser disabling cases, and then the population out of which these cases arise. It is also reasonable that the disabling ‘comorbidities’ of the BAP are therefore not essential features of this neurology, but rather, that they may be medical conditions worthy of amelioration, and that ameliorating these conditions would not change the underlying BAP way of thinking, but instead would allow it to flourish.
Minicolumns and The Brain
The neocortex (also known as the isocortex) is the 2 to 4mm tick top layer of the cerebral hemispheres and outer layer of the cerebral cortex. This layer is responsible for higher functions such as sensory perception, generation of motor commands, spatial reasoning, conscious thought, and language. The neocortex itself is divided into six layers, although there are no borders between these layers, and neuron dendrites and axons cross multiple layers.
The basic anatomical and physiological unit of the neocortex is the minicolumn. (Casanova 2006) As its name suggests, a minicolumn is a vertically aligned collection of cells and their projected connections. It is the smallest unit capable of information processing in the brain - resembling a mini-processor in function - receiving stimuli from elsewhere, processing information, and providing the capability of a response. Information is transmitted through the core of the minicolumn and is prevented from activating neighboring minicolumns by surrounding inhibitory fibers (interneuronal projections).
Pyramidal cells make up approximately 80% of the neurons of the cortex, and are the heart of the information processing capability of the minicolumn. Pyramidal cell somata (cell bodies) in layers III, V and VI are vertically oriented. Their dendrites and axon trees cross at least three layers, and in many cases all of the layers of the neocortex. They release glutamate as their neurotransmitter, and are the major excitatory component of the cortex.
Another component of minicolumns are various types of GABAergic inhibitory interneurons (i.e. neurons that produce GABA as their neurotransmitter), which tend to be aligned one on top of the other and modulate the activity of minicolumn pyramidal cells. Double bouquet cells are present in all layers, but are most dense in layers II and III. The axon bundles (long projections that conduct electrical impulses away from the cell body) of these cells are projected deep into the cortex from layer II to layer V, terminating on pyramidal cells as well as other inhibitory interneurons. They create a narrow vertical stream of inhibition through the cortex, as well as a vertically directed disinhibition of those pyramidal cells upon which the other inhibitory interneurons project. Other interneurons include Chandelier cells, which synapse directly onto the axon hillock (base of the axon projection from the cell body) of pyramidal cells, modulating cell output and participating in intra-columnar inhibition, and basket cells, which contacts the soma and dendrites of pyramidal cells, modulating input to these cells. Although these interneurons make up a small fraction of the total number of minicolumn cells, they play a significant role in finely tuning cortical information processing. A simplified representation of a minicolumn is thus of a processing core surrounded by a GABAergic interneuron circumferential zone of inhibitory and disinhibitory activity.
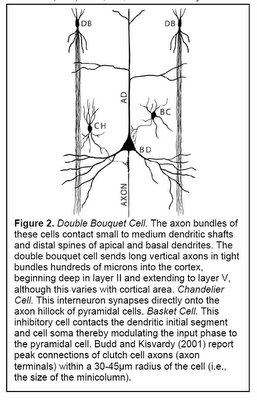
(Figure 2 Source – TMS Research Proposal in preparation for submission, pg 2, with permission)
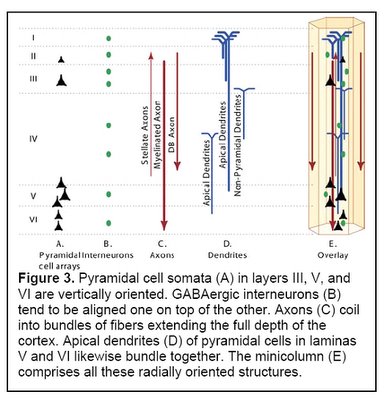
(Figure 3 Source – TMS Research proposal in preparation for submission, pg 3, with permission)
Minicolumns are the basic organizational unit of the cortex. It is an increase in the number of minicolumns, expanding the area covered by the isocortex, that is responsible for the historical process of encephalization, i.e. the increase in size of the brain to a degree greater than that expected based on body size (in modern humans, brain size is three to five times greater than expected when comparing to mammals of equal body mass) (Casanova - Big Brains Manuscript, in preparation for submission). A variable number of minicolumns are dynamically clustered with neighbouring minicolumns into macrocolumns, and from there in networks of macrocolumns and regions of functional differentiation within the brain. The minicolumn-macrocolumn relationship may be linked in part to both the termination of projections from the thalamus, which span a fixed distance and may serve to link together minicolumns that receive input from the same thalamocortical fibers, and by the effects of serotonin, changing columnar development in the cortex during brain development (Casanova 2006).
This minicolumn organizational structure conveys certain advantages. Through limiting connectivity within the brain (connectivity requires a significant amount of energy), the result is a reduction in metabolic expenditure. Instead of connecting every cell within the cortex to different brain regions, projections are organized into modules. Single cells project to target sites, and information gained or transmitted is transferred to other neurons within the same modules. Another advantage is plasticity, or the ability of the brain to naturally reorganize connections. Similarities between minicolumns suggest that they are capable of performing similar transformations on incoming information, allowing them to replace each other in case of injury or to adapt to changing requirements. This is not to suggest that all minicolumns are identical. There are variations within different regions, but these may arise from variations in input, output targets, interconnectivity, and inhibition (Casanova - Big Brains Manuscript, in preparation for submission).
So, what does this have to do with autism?
Analysis has determined that minicolumns in the brains of autistic individuals tend to be smaller in size, although with the same total number of cells per column (Casanova et al, 2002a and Casanova et al 2002b). Given that autistic brains tend to be larger than average, the results indicate that autistics also have a higher number of minicolumns. In addition, the neurons within these individual minicolumns tend to be reduced in size.
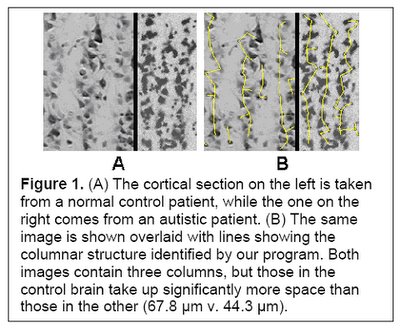
(Figure 1 Source – TMS Research proposal in preparation for submission, pg 2, with permission)
Smaller minicolumns would skew information processing (noise/signal) in favour of signal, potentially enhancing the ability to process stimuli that require discrimination, but also potentially at the expense of generalizing the salience of a particular stimulus. Smaller and more densely packed minicolumns could also allow for more complex information processing. As an example, the smaller minicolumns in the visual cortex may support added functionality, e.g. depth or color perception. This may be due to the overlap of neuronal projections allowed for when neuronal dendrites and axons remain the same size but the distance to neighbouring minicolumns is reduced (Casanova - Abnormalities Of Cortical Circuitry In The Brains Of Autistic Individuals).
These enhancements come at a cost. In autism, minicolumn size reductions result primarily from reductions in the peripheral zone of inhibitory and disinhibitory activity. The inhibitory fibers act to keep the stimuli within individual minicolumns, and the reduction in this space results in stimuli no longer being contained. Instead, they can overflow to adjacent minicolumns, providing an amplifier effect, which may explain hypersensitivity in some autistics. Thalamic input may also be amplified if thalamic terminal fields remain the same size and therefore result in more minicolumns per stimulated macrocolumn. Each minicolumn’s response to thalamic input is also modulated by the activity of neighbouring columns to a greater or lesser degree, allowing for gradations of response, so a reduction in GABAergic inhibitory activity could also result in a loss of inhibition and greater amplification (see Figure 1). Stimuli ‘spill’ and amplification could result in increased incidence of seizures in autistics.

(Casanova 2006, pg 3, with permission)
An additional cost is that imposed by a reduction in neuron size. The ability of a neuron to sustain a connection over distance is related to the size of its cell body. Smaller neurons result in a metabolic bias favouring shorter connections at the expense of both longer distance and inter-hemispheral connectivity. The result is that autistic brains have a bias towards local (intra-regional) over global (inter-regional) connectivity and processing. Short intra-regional processing functions include mathematical calculations and visual processing. Cognitive functions that require inter-regional processing would be less metabolically efficient, including language, face recognition, and joint attention (Casanova - Abnormalities Of Cortical Circuitry In The Brains Of Autistic Individuals).
The total number of minicolumns in the human brain is defined during the first forty days of fetal development. Symmetrical divisions of germinal cells determine the total number of minicolumns, and a second phase of asymmetrical divisions provides for cell migration into the cortex, with successive divisions determining the number of cells in each minicolumn. This is a complex process, defined by a large number of genes interacting with the environment. The result is that a higher number of minicolumns is not something that someone can ‘acquire’ or be vulnerable to within the post-natal period. It is possible for disruptions during the narrow window of fetal development (e.g. rubella, thalidomide, tuberous sclerosis) to also result in an increase in the number of minicolumns, by influencing this process (Casanova - Abnormalities Of Cortical Circuitry In The Brains Of Autistic Individuals; Casanova 2006).
One point that is important to make is that while narrower minicolumns is a factor in autism, evidence suggests that it is not related to mental retardation. In Down Syndrome, as an example, minicolumn width is normal, despite the smaller brain sizes of Down Syndrome patients.
Implications
Reduced minicolumn width appears to be a prerequisite for autism. But, the reported minicolumn widths found within autistic brains are still within the normal distribution of minicolumnar width, albeit at the tail end (Casanova 2006). This suggests to me that the existence of narrow minicolumns is not enough by itself to result in an ASD diagnosis. The key instead appears to be a reduction in inhibition within minicolumns, rather than width alone. Reduced width increases the consequences of reduced inhibition, but does not automatically cause it. In effect, a brain with narrower minicolumns may be less robust, and therefore more vulnerable to the complications that could come with deviation from the narrow tolerances within which the brain functions. In a brain with wider minicolumns, a loss of inhibition would not have as significant an impact, as minicolumnar width (and therefore distance between minicolumn information processing cores) would still exist to reduce intercolumnal spill, and thalamic projections would result in fewer minicolumns per macrocolumn to be affected.
It is a logical assumption that minicolumn development in MZ twins would be similar (same alleles, very minimal epigenetic differences during the early weeks of fetal development). But MZ twins do not always share the same ASD diagnosis. In cases in which one twin has an ASD diagnosis, the chance of the second twin also having an ASD diagnosis (not necessarily of the same severity) is 60%, and the chance of the second twin being within the broader autism phenotype is 92%. While ASD clearly has an inheritable component (the prevalence of ASD in the population is 0.6%, but within families with an ASD child, the chance of subsequent children being ASD is up to 10%), genetics alone is not sufficient to explain a diagnosis in all cases. This, combined with the fact that reduced autism minicolumn widths still fall within the normal range suggests to me that the issue in autism relates to loss of inhibition, to which autistics are more vulnerable than average, i.e. reduced minicolumnar width is a precondition for autism but a 'second hit' is still required. If that second hit were purely genetic, as distinct from epigenetic or environmental, then presumably both MZ twins should be similarly affected. This is clearly not the case a significant percentage of the time.
Support for this may come from studies of the brains of MZ twins discordant for autism. In Kates et al 2003, a comparison of MZ twins discordant for autism indicated that both the autistic and non-autistic (broader autism phenotype) twins showed no significant differences in cerebral gray matter. This suggests a structural similarity in MZ twins, and the possibility of a requirement for a ‘second hit’ to explain the discordant outcomes.
We can speculate on what that ‘second hit’ may be, and indeed the speculation as to a) whether a second hit exists and b) the causes, if any, are among the most controversial subjects of debate within the community of those linked by autism. I will look a bit more closely in this direction in a post in the near future.
What is clear from above though is that if narrow minicolumnar width is a) a precondition for ASD and b) not sufficient by itself to cause ASD, then this should have an impact on ASD research. If narrow minicolumns creates a vulnerability that wider minicolumns do not, then demonstrating lack of effect in those who are invulnerable does not demonstrate lack of effect in those who may be vulnerable. In many cases the correct population in causation analyses (e.g. epidemiological studies?) would not be the wider population, but instead the sub-section of the population who are actually vulnerable. And in looking for causes of autistic disability, we may want to look more closely at those events and/or stimuli that may reduce inhibition within the brain and/or further discourage global connectivity.
It is also a reasonable proposition is that a ‘cure’ for autism would not alter the underlying narrow minicolumnar structure of the brain. Instead, it would allow this structure to function more effectively, maintaining its strengths while reducing or eliminating the more disabling features of ASD. A cure would not change who one is, which is one of the fears of the neurodiversity community. Instead, it would allow autistic thought to function without the disabling comorbidities, serving to maintain neurodiversity and allowing it to flourish.
Update - Related post:
Autism and the Evolution of the Brain (Oct 13, 2006)
